AGC Biologics and Scifeon
AGC Biologics is a leading global Contract Development and Manufacturing Organization (CDMO), providing development and manufacture of mammalian and microbial-based therapeutic proteins, plasmid DNA (pDNA), viral vectors and genetically engineered cells.
In the summer of 2021, the Downstream Process Development department at AGC Biologic’s Danish laboratory facilities began collaborating with Scifeon on a software tool to help them improve their digital data handling. As the pilot implementation has successfully progressed, so has its scope, and the project is now attracting positive attention from other departments as well.

We spoke to Principal Scientist, Lars Schrøder, and Vice President, Manufacturing Science and Technology, Fredrik Nilsson, AGC Biologics, about the background for the project and their experience with Scifeon so far.
“Every time we move it manually, we need to countersign everything. That quickly adds 30% to any project.”
Fredrik Nilsson, Vice President
IMPROVED DATA INTEGRITY AND REDUCED TIME SPEND
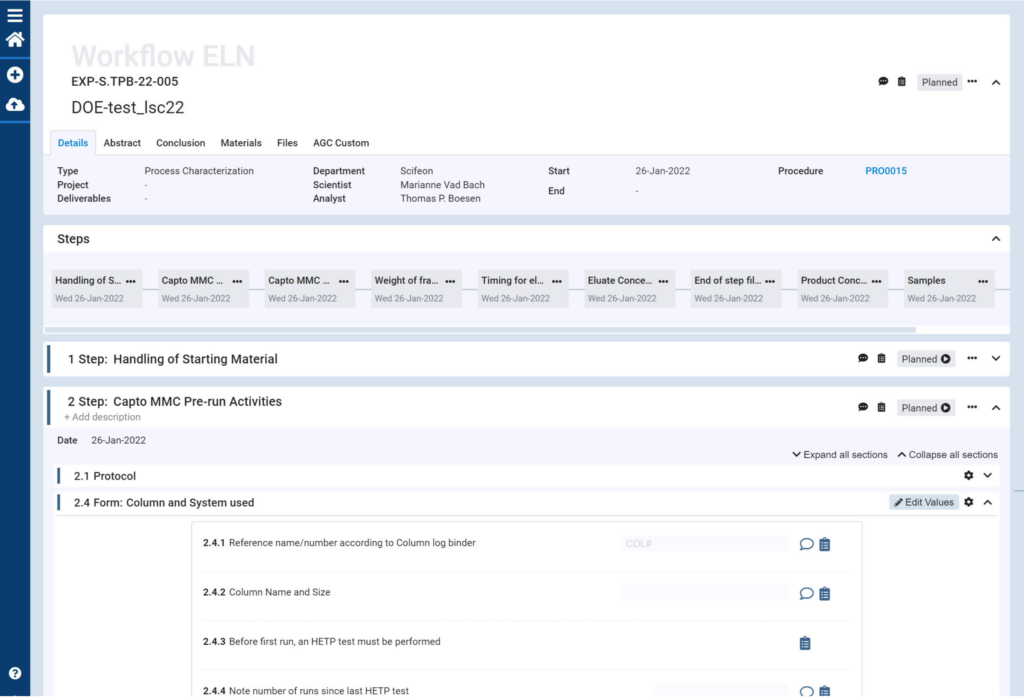
For AGC Biologics, the motivation for finding a better way to handle data was a practical question of time and data integrity. ‘Process characterization, that’s really where it all began. Process characterization generates lots of data that we used to keep track of and handle using pen and paper before entering it manually into Excel, and then copying it manually into other programs for further analysis’ explains Lars Schrøder. ‘It was just endless revisions and very repetitive work, and of course it introduced the risk of human error.’
Fredrik Nilsson concurs: ‘Our overarching concern is data integrity. We produce a lot of data that we need to collect, store, analyze and put into reports. Every time we move it manually, we need to countersign everything. That quickly adds 30% to any project in terms of hours used. If it’s a 5000-hour project, that amounts to a full-time employee.’
FURTHER BENEFITS DOWN THE ROAD
While the Scifeon platform offers additional benefits down the road, the question of saving time is, for now, still the deciding factor for Fredrik Nilsson: ‘Automatically collecting and storing our data makes it more accessible, more processable and more easily available for further analyses. It will make us able to start asking questions we didn’t know before, which will increase the value of our data. And of course, the end game is getting all our data into a format where we can use it with AI,’ he explains.
‘Hopefully it might also help us retain people, because they no longer have to do the very dull job of manually moving data around. But here and now the winning argument is to save hours on our data handling.’
“Automatically collecting and storing our data makes it more accessible, more processable and more easily available for further analyses. It will make us able to start asking questions we didn’t know before, which will increase the value of our data.”
Fredrik Nilsson, Vice President
FINDING THE RIGHT SOLUTION IS HARD WORK
Lars Schrøder began looking for a data handling solution for the Downstream Process Development department two years ago. ‘I started screening off-the shelf solutions. There are many out there, but not many good ones. It’s hard work, because it takes a lot of time to test them seriously. I ran a pilot test on one, which looked very fancy. But when it came to using it in real-life lab work, the problems began. The lab technicians and scientists in the lab had to change windows and input screens all the time. That’s no good when you’re working in a lab’ Lars Schrøder explains. ‘Spending 1000 hours on a piece of software that does everything you want but no one wants to use is a sobering experience,’ adds Fredrik Nilsson.
“There’s a lot of life science thinking built into the Scifeon platform. We don’t have to adapt our workflows to the system. We can adapt the system to our workflows.”
Lars Schrøder, Principal Scientist
CUSTOMIZATION IS KEY TO USEFULNESS
It’s a basic fact of life for any software solution: If it’s a hassle to use, no one will. And according to Fredrik Nilsson, ease of use is an absolute criticality when it comes to deploying a software platform intended to support lab work. ‘The ELN we are implementing with Scifeon is meant to be used by lab technicians. It needs to be totally intuitive and extremely easy to use. Just click one link and follow the flow. Now, it might not actually be the best way to build it – but it is the best way to get it used,’ he emphasizes. Lars Schrøder adds: ‘The creators of the Scifeon platform have a background in life sciences. You can tell that they know their way about a laboratory – it shows in the way they design their solutions. There’s a lot of life science thinking built into the Scifeon platform. We don’t have to adapt our workflows to the system. We can adapt the system to our workflows.’
AGILE IMPLEMENTATION GETS IT JUST RIGHT
The Scifeon approach to agile implementation means a grand vision can be chopped up and implemented in smaller, more manageable chunks. Building from a small project with a limited scope in one department, the Scifeon platform can then be scaled up and adapted to other departments as it proves its usefulness and user friendliness.
‘The agile development and implementation process was one of the reasons why we decided to use Scifeon,’ says Fredrik Nilsson. ‘We tell them what we want, and they are good at translating that into what we need.’ And if at first they don’t succeed, Scifeon simply gives it another go, as Lars Christensen explains: ‘The way the ELN was initially designed was too complex. So Scifeon broke it down into smaller, independent snippets. Using snippets as building blocks, we can now set up our ELN to match the exact requirements of any experiment. That has made it much easier to use.’ The agile implementation process also means a high degree of employee involvement on AGC Biologic’s side. ‘We are currently two scientists and a lab technician on our implementation team,’ says Lars Christensen. Once the solution goes live, the implementation team will be responsible for training their 30 colleagues in the Downstream Process Development department in how to use it.
For more information, please visit www.scifeon.com or contact Thomas Boesen at +45 5124 9454 or boesen@scifeon.com
